Lithium-ion batteries have a profound impact on daily life. Commercially available lithium-ion batteries using carbon negative electrodes are now close to their theoretical capacity, making it difficult to meet the increasing demands of portable electronic devices, electric vehicles and large-scale energy storage. Application requirements. Among the materials that can be used as a negative electrode for lithium batteries , metallic lithium has the largest theoretical energy density (3860 mAhg? 1 or 2061 mAhcm? 3) and the lowest electrochemical potential (3.04 V relative to a standard hydrogen electrode), which is the next generation of high-energy lithium. The best choice for batteries such as Li-S and Li-air batteries. However, metal lithium anodes are prone to dendrites in practical applications, and the problem of safety and stability is the focus of current research on metal lithium anodes.
Recently, Professor Cui Wei from the Department of Materials Science and Engineering at Stanford University published a review entitled "Reviving the lithium metal anode for high-energy batteries" at Nature Nano technology. The first systematic review of the current understanding of lithium metal anodes is emphasized. Recent advances in material design and advanced characterization methods, and provide a reference for the future research direction of lithium metal anodes.
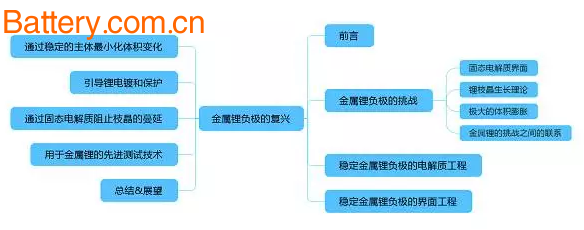
Overview map
1. The challenge of metal lithium anode
Before the practical use of metal lithium anodes, it is necessary to overcome the challenges in terms of safety and cycle stability. During the charge-discharge cycle, lithium is unevenly deposited to form dendrites and cause short-circuiting of the battery; at the same time, low coulombic efficiency and a gradual increase in lithium negative potential also lead to a sharp decrease in capacity. In order to solve these problems, it is necessary to have a deeper understanding of the interface chemistry, the behavior of lithium deposition, and the relationship between them.
1.1 Formation of solid electrolyte interface on lithium surface
The Solid Electrolyte Interface (SEI) is the focus of battery research. Since Li+/Li has a highly negative electrochemical potential, any electrolyte can be reduced on the lithium surface, which can be solved by passivating the SEI. However, the metal lithium anode has a high requirement for SEI. The SEI on lithium should have high lithium ion conductivity and good electron blocking ability, and the composition, morphology and ionic conductivity should be uniform. Due to the relatively large interface fluctuation during the cycle, SEI is also required to have good flexibility and even elasticity.
Lithium alkyl carbonates and ethers are two important electrolytes for lithium anodes. Improving lithium anodes in carbonate electrolytes is expected to replace traditional carbon anodes and greatly increase battery capacity. Development of lithium anodes in ether electrolytes In the long run, it will benefit the development of Li-S and Li-air batteries. More importantly, the mechanism of SEI formation of these two electrolyte systems is similar, and the discovery in one system can be applied to another system.
1.2 Lithium dendrite growth theory
When electroplating metals such as Cu, Ni and Zn at high currents, the cations are gradually depleted, breaking the electrical neutrality of the electrode surface to generate a space charge layer, resulting in uneven deposition of metals, and dendrite growth occurs. However, in lithium batteries, the formation mechanism of lithium dendrites is different, and the influence of interface chemistry needs to be considered. The reduction electrode of lithium has a relatively high potential and spontaneously forms an SEI layer on the surface. If the lithium ion conductivity of the SEI is not uniform, it will lead to uneven nucleation. In addition, volume changes in the cyclic process can cause cracks in the SEI, which in turn can exacerbate the uneven deposition of lithium. Lithium dendrite growth is a process of self-enhancement.
1.3 Great relative volume change
All electrode materials undergo volume changes during the charge and discharge cycle, and even commercial graphite electrodes have a 10% volume change. For metallic lithium, the volume change is greater because it has no body. From a practical point of view, the area capacity of a single-sided commercial electrode needs to reach 3 mAhcm?2, and for lithium there will be a volume change of 14.6 μm. This value will be even greater in the future, meaning that the movement of the lithium interface will reach tens of microns during the cycle.
1.4 The link between metal lithium challenges
Figure 1b summarizes the most important problems with metallic lithium anodes. In the process of the lithium plating, so that a huge volume expansion rupture SEI (Step 1), to promote the growth of lithium dendrite (Step 2) cracks. During the process of lithium stripping, the volume change further destroys the SEI layer, and the stripping from the junction breaks the electrical contact to form "dead" lithium (metal lithium is isolated from the substrate, step 3). After continuous cycling, the above process will The formation of a porous lithium electrode eventually occurs repeatedly, resulting in a sharp drop in capacity. A more detailed link is shown in Figure 1c. SEI fractures, chemical side reactions, dendrites and the formation of "dead" lithium eventually lead to serious safety problems and a drop in capacity.

Figure 1 Opportunities and challenges facing lithium metal anodes
a. Actual specific energy (pink) and energy density (blue) for gasoline, state of the art lithium-ion batteries , lithium metal/LOM batteries, Li-S and Li-air batteries
b. Schematic diagram of the lithium stripping/plating process. Step 1: Lithium plating expands the volume, SEI film cracks; Step 2: Mechanical plating causes lithium dendrite to grow from the crack; Step 3: Lithium stripping leads to the generation of isolated lithium, volume change causes SEI to further rupture; Step 4: Continuous cycling , causing 1-3 steps to occur repeatedly, leading to serious security problems and capacity decline
c. The connection between the different challenges of metallic lithium anodes stems from high reactivity and extreme relative volume changes.
2. Electrolyte engineering for stabilizing lithium metal anode
Electrolyte additives have been used to improve the performance of lithium anodes. These additives can decompose, polymerize or adsorb on the surface of lithium, modify the physicochemical properties of SEI, and regulate the current distribution during lithium deposition. The presence of additives, sometimes on the order of ppm, can also change the deposition morphology and cycle efficiency. Typical additives are gas molecules (CO2, SO2, N2O), 2-methylfuran, organic aromatic compounds, and various surfactants.
2.1 fluorochemicals
The addition of a small amount of HF and H2O to the carbonate electrolyte forms a dense and uniform LiF/Li2O bilayer on the surface of the lithium, which smoothes the deposition of lithium. However, Coulomb's efficiency is not high, and the protection disappears after several cycles. This is a problem with this method. Similar problems exist with fluorine salts such as LiPF6, (C2H5)4NF(HF)4, and LiF. Vinyl fluorocarbonate is a thin film forming additive that forms a soft film on the surface of lithium, inhibits the formation of lithium dendrites, and improves the coulombic efficiency.
2.2 Self-repairing electrostatic field
By adding additives such as Cs+ and Rb+ to the carbonate electrolyte, the formation of lithium dendrites can be avoided by the "self-repairing electrostatic field" mechanism. If the metal ion (M+) additive has a lower reduction potential than lithium, M+ will adsorb on the lithium surface without being reduced during lithium deposition. If uneven lithium deposition occurs, the accumulation of charge at the bumps will attract more M+ to form an electrostatic field. The positively charged electric field will repel lithium ions and reduce the spread of the bumps, thereby improving the deposition quality of lithium.
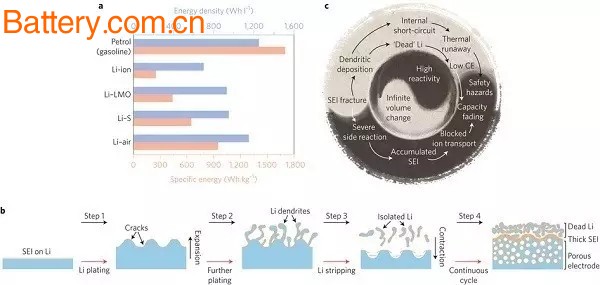
Figure 2 Effect of different electrolyte additives
a. An illustration of a lithium deposition process based on self-healing electrostatic field theory. M+ will adsorb on the bump to form an electrostatic field, repel lithium ions and slow the growth of the bumps.
b. Deposition of lithium in 1M LiPF6/propylene carbonate without additive at a current density of 0.1 mAcm?2
c. Deposition of lithium in 1M with 0.05M CsPF6 LiPF6/propylene carbonate at a current density of 0.1 mAcm?2
d. SEM image of lithium metal lithium surface in 7MLiTFSI (DOL/DME) electrolyte after 280 cycles, it can be seen that there is no dendrite formation on the surface
e. Surface topography and optical photos of lithium deposited on copper in 1MLiPF6/PC (left) and 4MLiFSI/DME (right) at 1.0 mAcm?2 current density
Side Sealing Flat bags are made from Food Grade Virgin material. environmentally friendly and hygienic. They are water-proof, Moisture-proof, strong toughness, tear resistance, good for storage and usually for packing sugar, biscuits, candy, coffee bin, fruits , drinks or other foods. Normally width from 3 inch to 12 inch. Length from 7 inch to 22 inch. Material could be in HDPE or LDPE.


Flat Poly Bag, Flat Plastic Bag, Side Sealing Bag, Side Seal Bag, Four Side Seal Pouch, Side Sealing Plastic Bag
BILLION PLASTIC MANUFACTURING CO.,LTD, JIANGMEN , https://www.jmfoodpackagingbag.com